Abstract
Background: FL is associated with frequent relapses and decreasing progression-free intervals with successive lines of conventional therapy. Later-line treatments may be less effective due to refractory disease. Mosunetuzumab (Mosun) is a CD20xCD3 bispecific antibody (Ab) that redirects T cells to eliminate malignant B cells. In the dose-escalation phase of an ongoing Phase I/II study (NCT02500407), Mosun was highly active and well tolerated in R/R FL patients (pts) who had received ≥2 prior lines of therapy (3L+ R/R FL) when given IV with Cycle (C) 1 step-up dosing for mitigation of cytokine release syndrome (CRS; Assouline et al. ASH 2020). We present pivotal data from the same study from a large expansion cohort of 3L+ R/R FL pts who received Mosun monotherapy at the recommended Phase II dose (1/2/60/30mg).
Methods: 3L+ R/R FL pts were enrolled into a single-arm, pivotal expansion cohort. All pts had FL (Grade [Gr] 1-3a), ECOG PS ≤1, and were R/R to ≥2 prior lines of therapy including an anti (a)-CD20 Ab and an alkylator. Mosun was given IV in 21-day cycles with step-up dosing in C1 (C1 Day [D]1: 1mg; C1D8: 2mg; C1D15 and C2D1: 60mg; D1 C3+: 30mg). Pts who achieved a complete response (CR) by C8 discontinued therapy; those with a partial response or stable disease continued treatment for a total of 17 cycles, unless disease progression (PD) or unacceptable toxicity occurred. The primary endpoint was CR (as best response) rate by PET/CT assessed by an independent review facility (IRF) using standard response criteria (Cheson et al. J Clin Oncol 2007). No mandatory hospitalization was required.
Results: A total of 90 pts were enrolled (median age: 60 years, range: 29-90; 61.1% male). At entry, 76.7% had stage III or IV disease and 44.4% had FLIPI 3-5. Median number of prior lines of therapy was 3 (range: 2-10). In addition to aCD20 Abs and alkylators (all pts), prior cancer therapies included anthracyclines (82.2%), ASCT (21.1%), PI3K inhibitors (18.9%), IMiDs (14.4%), BTK inhibitors (6.7%), and CAR-Ts (3.3%). 68.9% of pts were refractory to their last therapy, 78.9% to any prior aCD20 Ab, and 53.3% to any prior aCD20 Ab and an alkylator (double refractory). 52.2% had PD within 24 months from the start of initial therapy (POD24).
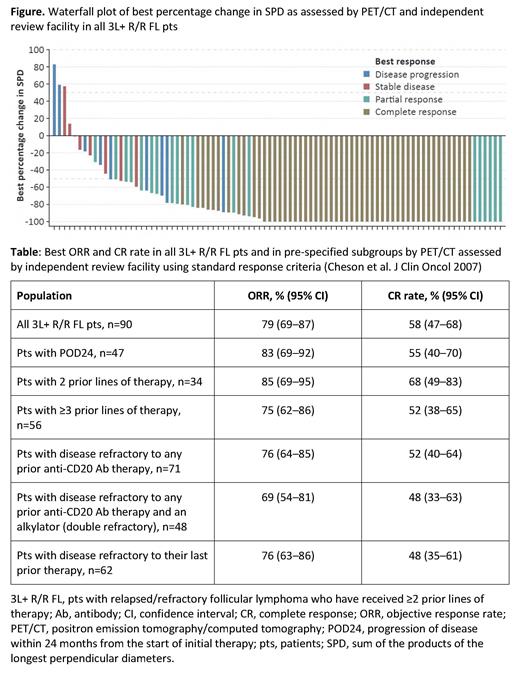
As of March 15, 2021, median time on study was 12.9 months (range: 2.0-22.1). Anti-tumor activity was seen in most pts (Figure). Best objective response (ORR) and CR rates by IRF were 78.9% (71/90 pts) and 57.8% (52/90), respectively (Table); the median time to first response was 1.4 months. Best ORR and CR rates were generally consistent in pre-specified subgroups (Table), including POD24 (ORR: 83%; CR: 55%) and double-refractory pts (ORR: 69%; CR: 48%). Median duration of objective response and CR was not reached; 12-month event-free rates after first response were 65.4% (95% CI: 52.6-78.1%) in all responders and 80.1% (95% CI: 67.4-92.7%) in CR pts. Median PFS was 17.9 months (95% CI: 12.0-not estimable).
CRS (Lee et al. Biol Blood Marrow Transplant 2019) was the most common adverse event (AE; 44.4% of pts). CRS was mostly confined to C1 and generally low Gr (Gr 1: 25.6%; Gr 2: 16.7%). High Gr CRS was uncommon (Gr 3: 1 patient; Gr 4: 1 patient with FL in leukemic phase); no Gr 5 CRS occurred. In the 40 pts with CRS, tocilizumab was used in 7 pts and corticosteroids in 9 pts; all events resolved after a median duration of 3 days. Other common (≥20%) AEs were fatigue (36.7%), headache (31.1%), neutropenia and pyrexia (28.9% each), hypophosphatemia (22.2%), and pruritus (21.1%). Common (≥5%) Gr 3-4 AEs (66.7% overall) were neutropenia (26.6%), hypophosphatemia (13.3%), hyperglycemia and anemia (7.8% each), and elevated ALT (5.6%). Gr 3 neurologic events were uncommon (4.4%) and no Gr 4-5 events occurred. Common (≥5%) SAEs (45.6% overall) were CRS (23.3%, including 2.2% Gr 3-4 CRS). Two Gr 5 (fatal) AEs occurred (malignant neoplasm progression and unexplained death; both considered unrelated to Mosun by investigators). AEs leading to Mosun discontinuation were uncommon (4 pts; 4.4%).
Conclusions: Mosun induces deep and durable remissions in 3L+ R/R FL pts, including those with POD24 and/or double-refractory disease. High response rates are achieved (ORR: 78.9%; CR: 57.8%) and maintained for ≥12 months in the majority of pts. Mosun has a manageable safety profile, with C1 step-up dosing effectively mitigating CRS, enabling treatment without mandatory hospitalization. Mosun represents an active new therapy for 3L+ R/R FL.
Budde: Merck, Inc: Research Funding; Amgen: Research Funding; AstraZeneca: Research Funding; Mustang Bio: Research Funding; Novartis: Consultancy; Gilead: Consultancy; F. Hoffmann-La Roche Ltd: Consultancy; BeiGene: Consultancy; Genentech, Inc.: Consultancy. Sehn: Novartis: Consultancy; Genmab: Consultancy; Debiopharm: Consultancy. Matasar: Teva: Consultancy; Rocket Medical: Consultancy, Research Funding; Bayer: Consultancy, Honoraria, Research Funding; Merck: Consultancy; Juno Therapeutics: Consultancy; Takeda: Consultancy, Honoraria; Pharmacyclics: Honoraria, Research Funding; Genentech, Inc.: Consultancy, Honoraria, Research Funding; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; TG Therapeutics: Consultancy, Honoraria; Merck Sharp & Dohme: Current holder of individual stocks in a privately-held company; Memorial Sloan Kettering Cancer Center: Current Employment; Daiichi Sankyo: Consultancy; Janssen: Honoraria, Research Funding; IGM Biosciences: Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; ImmunoVaccine Technologies: Consultancy, Honoraria, Research Funding; GlaxoSmithKline: Honoraria, Research Funding. Schuster: Novartis: Consultancy, Honoraria, Patents & Royalties, Research Funding; Abbvie: Consultancy, Research Funding; Pharmacyclics: Research Funding; Merck: Research Funding; Genentech/Roche: Consultancy, Research Funding; Tessa Theraputics: Consultancy; Loxo Oncology: Consultancy; Juno Theraputics: Consultancy, Research Funding; BeiGene: Consultancy; Alimera Sciences: Consultancy; Acerta Pharma/AstraZeneca: Consultancy; Adaptive Biotechnologies: Research Funding; Incyte: Research Funding; TG Theraputics: Research Funding; Nordic Nanovector: Consultancy; Celgene: Consultancy, Honoraria, Research Funding. Assouline: Pfizer: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; Takeda: Research Funding; Roche/Genentech: Research Funding; Jewish General Hospital, Montreal, Quebec: Current Employment; Eli Lilly: Research Funding; Novartis: Honoraria, Research Funding; Amgen: Current equity holder in publicly-traded company, Research Funding; Gilead: Speakers Bureau; Johnson&Johnson: Current equity holder in publicly-traded company. Giri: Royal Adelaide Hospital: Current Employment. Kuruvilla: AbbVie: Honoraria; Amgen: Honoraria; Antengene: Honoraria; Janssen: Honoraria, Research Funding; Merck: Honoraria; AstraZeneca: Honoraria, Research Funding; Medison Ventures: Honoraria; TG Therapeutics: Honoraria; Seattle Genetics: Honoraria; Incyte: Honoraria; Karyopharm: Honoraria, Other: Data and Safety Monitoring Board; Roche: Honoraria, Research Funding; Gilead: Honoraria; Pfizer: Honoraria; Novartis: Honoraria; BMS: Honoraria. Canales: iQone: Honoraria; Novartis: Consultancy, Honoraria; Sanofi: Consultancy; Celgene/Bristol-Myers Squibb: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Incyte: Consultancy; Takeda: Consultancy, Honoraria, Speakers Bureau; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Speakers Bureau; Sandoz: Honoraria, Speakers Bureau; Eusa Pharma: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Speakers Bureau; Gilead/Kite: Consultancy, Honoraria. Dietrich: University Hospital Heidelberg: Current Employment; F. Hoffmann-La Roche Ltd: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; KITE/Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Fay: St Vincent's Hosptial, Sydney, Australia: Current Employment. Ku: Roche: Consultancy; Antegene: Consultancy; Genor Biopharma: Consultancy. Nastoupil: Epizyme: Honoraria, Research Funding; MorphoSys: Honoraria; Janssen: Honoraria, Research Funding; ADC Therapeutics: Honoraria; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; IGM Biosciences: Research Funding; Caribou Biosciences: Research Funding; Gilead/Kite: Honoraria, Research Funding; Denovo Pharma: Other: DSMC; Takeda: Honoraria, Other: DSMC, Research Funding; Novartis: Honoraria, Research Funding; Bayer: Honoraria; TG Therapeutics: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding. Wei: Genentech, Inc.: Current Employment; F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Yin: Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Doral: Genentech, Inc.: Current Employment; F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Li: Genentech, Inc.: Current Employment, Current holder of individual stocks in a privately-held company. Huang: F. Hoffmann-La Roche Ltd: Current Employment. Negricea: F. Hoffmann-La Roche Ltd: Current Employment, Current equity holder in publicly-traded company. Penuel: Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company. O'Hear: F. Hoffmann-La Roche Ltd: Current holder of individual stocks in a privately-held company; Genentech, Inc.: Current Employment. Bartlett: Pharmacyclics: Research Funding; Genentech, Inc./F. Hoffmann-La Roche Ltd: Membership on an entity's Board of Directors or advisory committees, Research Funding; Millennium: Research Funding; Merck: Research Funding; Kite Pharma: Research Funding; Janssen: Research Funding; Forty Seven: Research Funding; Celgene: Research Funding; Bristol-Myers Squibb: Research Funding; Autolus: Research Funding; Affimed: Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Washington University School of Medicine: Current Employment; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Mosunetuzumab is a CD20xCD3 bispecific antibody that redirects T cells to engage and eliminate malignant B cells. Mosunetuzumab is an investigational agent.